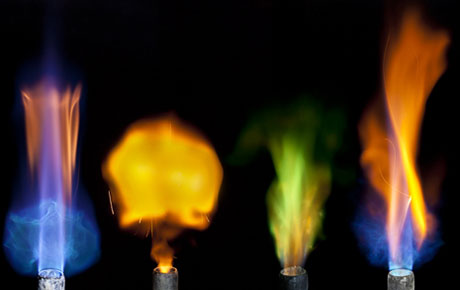
IMAGE: Photograph by Chris Hoover/Modernist Cuisine LLC, via The New Yorker.
This image, one of a dozen in a New Yorker slideshow selection from The Photography of Modernist Cuisine, shows the very different flames produced by different salts, sprinkled over a a burner. Ordinary table salt (sodium chloride) created the two flames on the left, followed by copper sulphate (a mineral salt and anti-caking agent) and potassium phosphate, which, depending on its molecular weight, can be found in Gatorade, non-dairy creamer, or ReddiWhip.
Seeing it reminded me of something Darren O’Donnell of Mammalian Diving Reflex said at Foodprint Toronto about the art of reading fire. The context was a series called The Beautiful and Hungry City, in which Mammalian Diving Reflex invited people whose jobs are essential to the city, but aren’t typically considered “creative,” to talk about the overlooked artistic aspects of their work at a community dinner. Speakers included a paramedic and an accredited Tagalog court interpreter, as well as firefighter Stacy Hannah, who “talked about the beauty of the fire and the different colors that different materials have when they burn […] and the choreography of fire.”
As it turns out, firefighters are typically given an extensive colour vision test (typically the Farnsworth-Munsell 100 Hue Test, which you can try an abbreviated version of online), and trained to read the colour of both smoke and flames for clues as to a fire’s nature and future behaviour. Flame colour can give clues as to the material that is burning and the fire’s temperature: for example, as an article advising colour-blind would-be firefighters explains, “a fire with a temperature near 977 degrees Fahrenheit will have a barely visible reddish flame, while a fire with a temperatures near 1,830 degrees will have a cherry-red flame.”
Australian firefighter Shan Raffel, writing in Firefighter Nation magazine, points that out that, in real life, most burning structures will be filled with multiple different fuel types, and so the most useful information to be gathered from flames is about the efficiency and type of combustion occurring. Using the example of particle board, Raffel explains that “when the air supply is good, the flame is yellow. When the oxygen concentration is reduced, the flame becomes a reddish-orange color. ”
The shape or form of the flame can also give an indication of the type of combustion occurring. The reddish-orange flames are often turbulent with a short-wave form. The ignition of accumulated pyrolysis products (aka combustible gases) produces a very light-yellow flame (sometimes almost clear). Amazingly in this case, the wave form is larger and the flames seem very slow.
In any case, it occurs to me that, in some ways, you could say that the art of cooking is also to know food by how it burns—or, better, how it is transformed by fire.

